
9
THE CAMLOG
®
IMPLANT SYSTEM
SYSTEM INFORMATION
CAMLOG
®
IMPLANTATSYSTEM
NON
STERILE
GENERAL SAFETY INSTRUCTIONS AND WARNINGS
The descriptions in this product catalog are not sufficient
to allow immediate use of the CAMLOG
®
Implant System.
Instruction by a surgeon experienced in using the CAMLOG
®
Implant System is strongly recommended.
An improper procedure during use of the CAMLOG
®
Products can lead to failures, implant damage, bone loss or
unsatisfactory esthetic results. The products may be used only
by dentists, surgeons and dental technicians trained in the
implant system. Use of these products requires specialized
knowledge and experience in implant dentistry. Detailed
information on the choice of suitable implants, prosthetic
components, treatment planning and application is contained
in the user information. Your local representative will be
happy to provide you with information.
Consulting and technical advice on the use of our products
is provided orally, in writing, by electronic media and/or by
demonstration. The provided information represents the
current state of science and technology known to us at the
point in time that the product is placed on the market.
This does not exempt the user from the responsibility to
personally test the product for suitability for the intended
purpose, indication and procedure. Handling and use of
the product take place outside of our control and is the
direct responsibility of the user. All liability for damages
resulting from such use is waived. ALTATEC GmbH/CAMLOG
Biotechnologies AG does not warrant nor provide replacement
services when non-system components are used.
The CAMLOG
®
Implant System is part of a comprehensive
treatment concept and must be used only with the pertinent
original parts and tools according to the suggestions and
instructions for use provided by the manufacturer. All
components of the CAMLOG
®
Implant System are matched
precisely to one another. The use of third party components
can affect the function and safety of the system.
Instruments and system components are designed for specific
implant and prosthetic lines and implant diameters. Their
use with any other implant or prosthetic product lines or
a different diameter can lead to the mechanical failure of
system components, tissue injury, or esthetically unsatisfactory
results. Because of this, some implant and prosthetic product
lines have some dedicated components and tools. Pay
attention to the color markings when choosing instruments
for the required implant diameter.
Products intended for single use must not be reused because
safe preparation and/or functional safety cannot be ensured.
Within the bounds of our sales and delivery terms, we
guarantee the flawless quality of our products.
Because of the small sizes involved, it may happen that a
product is swallowed and/or aspirated. Aspiration can lead to
dyspnea and in the worst case to asphyxiation. For this reason,
products must be secured in general against aspiration and
swallowing during intraoral use.
Where indications are listed for a particular product, it should
be noted that any indications that are not listed are in fact
contraindicated.
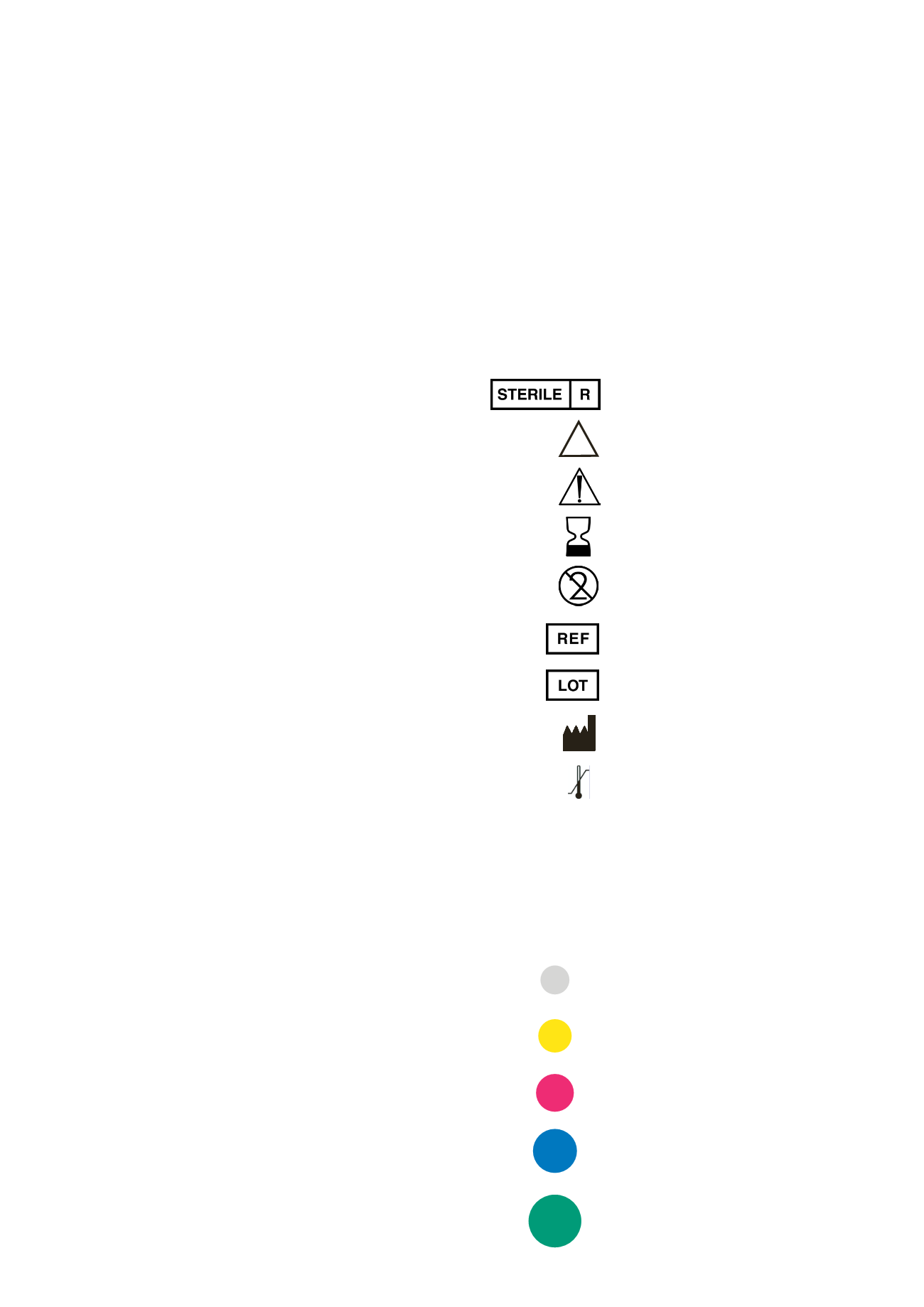
Sterilization using irradiation
Non-sterile
Caution, consult accompanying documents
Use by
Do not reuse
Article number
Lot number
Manufacturer
Temperature limitation
Caution: US Federal law restricts this device to
sale by or on the order of a dentist or physician.
Sterile packaged products must be kept dry, out of direct
sunlight and at room temperature. The packaging must be
checked before opening for damage and for the expiration
date and should only be opened immediately before the
products are used.
Not all products are available in all countries.
PACKAGING UNITS
If not otherwise specified, the packaging unit is 1 each.
EXPLANATION OF SYMBOLS/CHARACTERS ON
LABELS/PRODUCT LABELS AND PACKAGE INSERTS
Rx only
COLOR-CODING OF THE SURGICAL AND
PROSTHETICAL CAMLOG
®
PRODUCTS
Color
Diameter
gray
3.3 mm
yellow 3.8 mm
red
4.3 mm
blue
5.0 mm
green 6.0 mm



















